

δ7Li for the dissolved load decreases with increasing levels of dissolved silicon, and the saturation index of secondary minerals, which suggests that δ7Li decreases with increasing chemical weathering. In turn, the δ7Li value of the dissolved load is always greater than that of the bedload, ranging from 17.0 to 43.7‰. For example, hydrogen, the lightest element, has three isotopes with mass numbers 1, 2, and 3. The δ7Li value of the suspended load is always lower than that of the bedload due to preferential retention of 6Li in secondary minerals during weathering. Every chemical element has one or more radioactive isotopes. In contrast, in non-glacial rivers, uranium activity ratios increase with distance downstream due to continued weathering in soils and of bedrock. Activity ratios in glacial rivers decrease with distance from the glacial source due to input from non-glacial tributaries which have high levels of dissolved uranium and lower activity ratios.
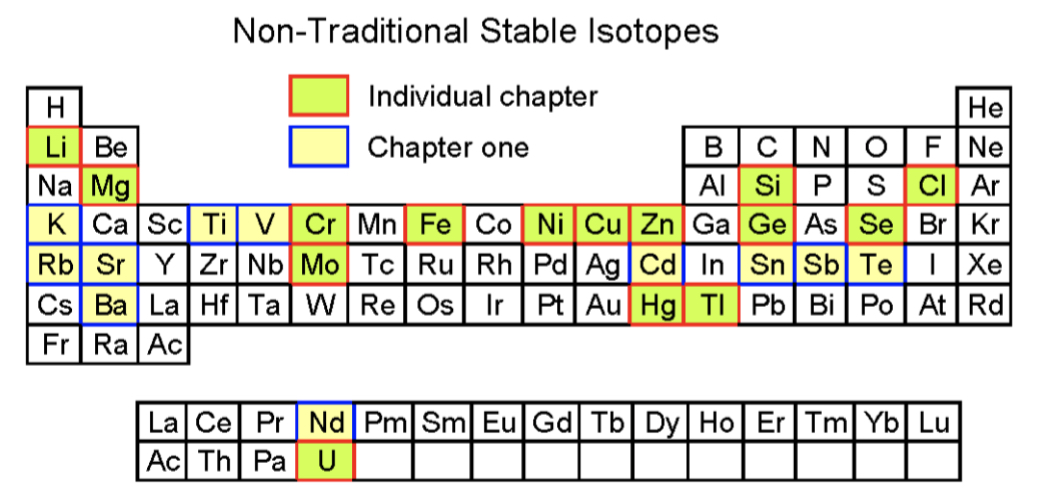
Some elements have no stable isotopes and eventually decay to other elements. The highest (234U/238U) values are found in glacier-fed rivers, and can be attributed to α-recoil effects, as grinding by glaciers locally enhances rates of physical weathering. Although most of the known elements have at least one isotope whose atomic nucleus is stable indefinitely, all elements have isotopes that are unstable and disintegrate, or decay, at measurable rates by emitting radiation. Uranium activity ratios, (234U/238U), are close to secular equilibrium in the suspended and bedloads, but all dissolved load samples show values greater than unity, ranging from 1.13 to 2.41. Chemical erosion rates range from 45 to 91 t/km2/yr, with lower rates being associated with glacier-fed rivers. Physical erosion rates range from 920 to 2084 t/km2/yr, with the higher values associated with glacier-fed rivers. This study presents U and Li isotope and major and trace element data for the dissolved load, suspended particulates and bedload for Icelandic rivers draining predominantly basaltic catchments. Earth and Planetary Science Letters, 251(1-2) pp. Period, 7, Boiling point Block, d, Density (g cm3) Atomic number, 89, Relative atomic mass State at 20C, Solid, Key isotopes Electron configuration, Rn. Riverine behaviour of uranium and lithium isotopes in an actively glaciated basaltic terrain.


 0 kommentar(er)
0 kommentar(er)
